For our community, especially young adults facing synovial sarcoma, the journey is often filled with uncertainty. When standard treatments for advanced disease have reached their limit, the need for new hope is deeply felt. Today, we want to talk about a landmark new therapy called afamitresgene autoleucel (Tecelra)—a living me dicine that teaches a patient’s own body to fight the cancer.
Training Your Own Immune System to Fight
For decades, the main approach for advanced synovial sarcoma has been chemotherapy. While it can be a powerful tool, the search has always been for a more targeted approach. Tecelra represents a huge leap forward. It’s the very first T-cell receptor (TCR) therapy approved for a solid tumor.
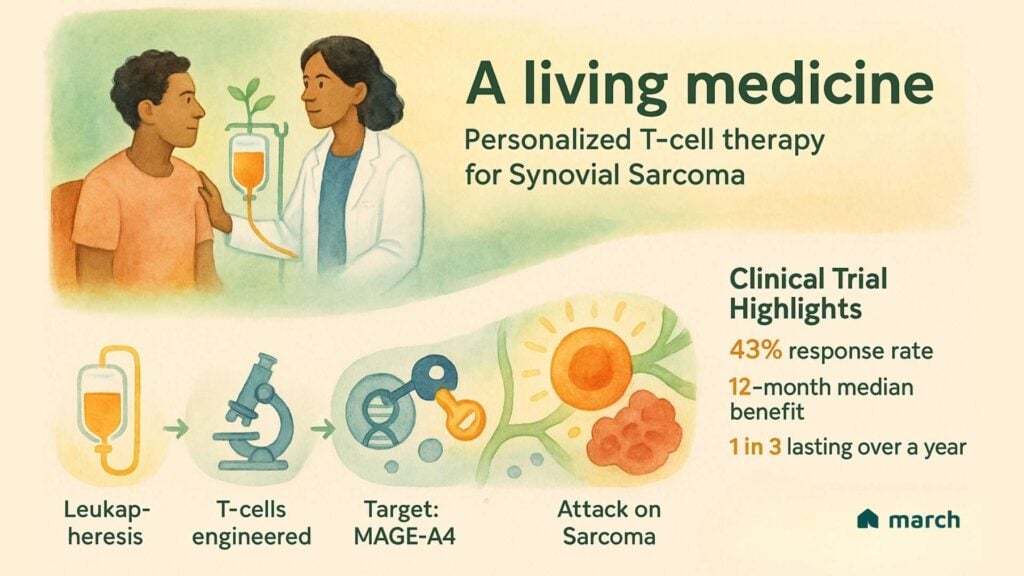
The concept behind it is both brilliant and deeply personal. It begins by collecting a patient’s own T-cells, the natural soldiers of the immune system, through a process called leukapheresis. In a lab, these cells are genetically engineered with a new targeting system. This system is trained to hunt for a specific “flag” called the MAGE-A4 protein, which is often found on synovial sarcoma cells but has limited expression in normal tissues.
What makes it so precise is that the attack only happens when there’s a “secret handshake”—the T-cells must see both the MAGE-A4 flag and a partner molecule called HLA-A*02. This high level of personalization means the therapy is only for patients who have this specific genetic profile, which is confirmed by blood and tumor tests before treatment.
A Landmark Moment: Hope from Clinical Trials
This approach moved from theory to reality in the SPEARHEAD-1 clinical trial, offering real hope to people with advanced synovial sarcoma who had few remaining options. The results were incredibly encouraging for our community:
- The therapy shrank tumors in 43% of participants.
- For those who responded, the effects were often lasting, with a median duration of response of 12 months.
- Most powerfully, 39% of the patients who responded were still seeing that benefit at the one-year mark.
For a community that has been waiting for new options for over a decade, these results are a monumental step forward.
Walking the Path with Realism
We walk this path with you, holding both hope and honesty. This is a powerful and complex therapy, and the journey is intensive. It involves collecting cells, undergoing preparatory (lymphodepleting) chemotherapy to make room for the new “soldier” cells, and a hospital stay for the single-dose infusion and close monitoring.
Because it so powerfully activates the immune system, Tecelra comes with a U.S. Boxed Warning for significant side effects, most notably Cytokine Release Syndrome (CRS). CRS occurred in 75% of patients in the clinical trial, with symptoms like fever, nausea, and low blood pressure. While manageable, this risk requires that the therapy only be given at specialized, authorized treatment centers with expertly trained teams who can monitor patients for at least 7 days after infusion.
Marching Forward Together
The arrival of Tecelra is a new dawn, but it’s not the end of the journey. As we watch this new frontier unfold, we are committed to providing you with clear, trustworthy information. Your hope and your experiences fuel this forward march, and on this journey of discovery, you are not alone.
Sources:
National Cancer Institute. (2024, May 17). Synovial Sarcoma Treatment (PDQ®)–Patient Version.
National Cancer Institute. (2023, June 30). FDA Approves First Cellular Therapy for a Solid Tumor Cancer.
Grønniger, E., et al. (2023). Unmet needs in synovial sarcoma and epithelioid sarcoma: a survey of sarcoma experts. Frontiers in Oncology.
U.S. Food & Drug Administration (FDA). (2024, October 4). FDA approves first cellular therapy to treat a solid tumor.
Cancer Research Institute. (2024, October 4). FDA Approves First T-Cell Receptor (TCR) T-Cell Therapy for Solid Tumors.
Adaptimmune Therapeutics plc. (2024, October 4). Adaptimmune’s Afami-cel Receives U.S. FDA Accelerated Approval for the Treatment of Advanced Synovial Sarcoma. [Press Release].
The Medical Letter. (2024). Afamitresgene Autoleucel (Tecelra) for Synovial Sarcoma. The Medical Letter on Drugs and Therapeutics.
TECELRA (afamitresgene autoleucel). (n.d.). Preparing for TECELRA Treatment.
Clinical Trials Arena. (2024, October 7). Adaptimmune’s afami-cel bags FDA approval for synovial sarcoma.
Aetna Inc. (2024). Clinical Policy Bulletin: Afamitresgene Autoleucel (Tecelra).
U.S. Food & Drug Administration (FDA). (2024). Highlights of Prescribing Information: TECELRA (afamitresgene autoleucel).
TECELRA (afamitresgene autoleucel). (n.d.). How TECELRA Works.
TECELRA (afamitresgene autoleucel). (n.d.). Important Safety Information for TECELRA.
U.S. Food & Drug Administration (FDA). (2024, October 4). Package Insert – TECELRA.
College of American Pathologists (CAP). (2024, November). A companion diagnostic for a new sarcoma T-cell therapy. CAP TODAY.
Drugs.com. (2024). Tecelra (Afamitresgene Autoleucel) Professional Patient Advice.
TECELRA (afamitresgene autoleucel). (n.d.). The TECELRA Treatment Journey.
Stanford Medicine. (2024, October 4). Stanford Medicine among first to offer newly approved cell therapy for rare soft-tissue cancer.
U.S. Food & Drug Administration (FDA). (n.d.). TECELRA (afamitresgene autoleucel) REMS.
TECELRA (afamitresgene autoleucel). (n.d.). Safety Profile.
TECELRA (afamitresgene autoleucel). (n.d.). Find a Treatment Center.
National Cancer Institute. (n.d.). NCI Dictionary of Cancer Terms: Cytokine Release Syndrome.