For so many in our Hemophilia B community, life has been measured by infusions. We know the constant vigilance required, the planning, the needle sticks, and the worry about breakthrough bleeds that have long been part of the standard prophylactic care. While life-saving, this treatment also carries a significant burden [1, 2]. The dream has always been for something more, something that could fundamentally change this daily reality. Today, we want to walk together through the story of one such leap forward: gene therapy.
It’s a field filled with immense promise, complex science, and real-world challenges. To that end, by exploring the journey of a specific therapy, we can better understand both the incredible hope on the horizon and the hurdles we face as a community.
Living with Hemophilia B
At its core, Hemophilia B is caused by the body’s inability to produce enough of a specific clotting protein called Factor IX (FIX), due to an error in the F9 gene [3, 4]. For decades, the standard of care has been to replace this missing factor through regular intravenous (IV) infusions, a treatment known as prophylaxis [2, 5]. While this has been revolutionary in preventing joint damage and life-threatening bleeds, it requires a demanding, lifelong commitment [2, 5].
Therefore, this is where the promise of gene therapy comes in a strategy designed not just to treat symptoms, but to address the root cause by giving the body a new, working instruction manual [4, 6].
How Gene Therapy Works
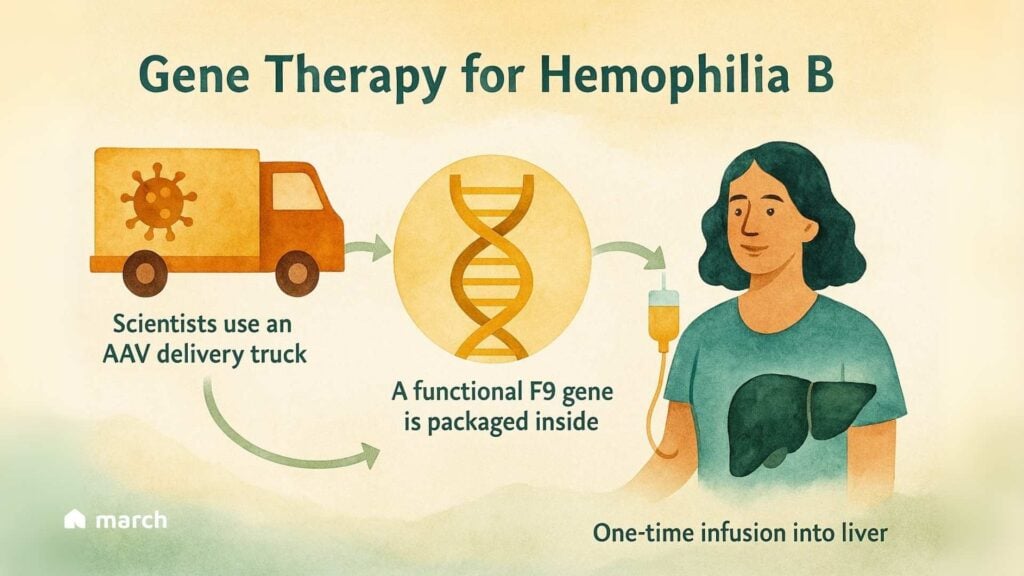
The concept, at its heart, is beautifully straightforward. Think of it like a special delivery service for your cells.
The Delivery Truck: Scientists use a specially engineered, harmless virus called an Adeno-Associated Virus (AAV) as a delivery vehicle. Its own viral genes are removed, so it can’t cause illness [4, 6].
The Package: Inside this AAV truck is a precious package: a functional copy of the F9 gene [4, 6].
The Destination: After a single, one-time IV infusion, these delivery trucks travel to the liver—the body’s natural factory for clotting factors [4, 6].
The goal is to turn the patient’s own liver into a “biofactory” that can produce its own Factor IX, potentially for years, from just one treatment [4, 6]. This could mean freedom from the relentless schedule of infusions.
A Case Study for Fidanacogene Elaparvovec (Beqvez)
This isn’t just a theory; it’s a reality that has been tested. Fidanacogene elaparvovec (known as Beqvez) is a gene therapy for Hemophilia B that brought this science to life. It uses a clever “supercharged” version of the Factor IX gene, called FIX-Padua, which produces a more potent protein [7]. This allowed for a lower dose of the “delivery truck,” a key goal for safety [7].
The clinical trial results were a source of incredible hope for our community:
- A 71% reduction in the annual bleeding rate compared to prior prophylaxis [7].
- Most powerfully, 60% of participants experienced zero bleeds after treatment [7].
- The vast majority of men in the study were able to stop their routine prophylactic infusions [7].
This is the kind of progress that can truly change lives. At the same time, we walk this path with you, holding both hope and honesty. The journey to bring a new therapy to market is incredibly complex. Despite the scientific success, Beqvez was withdrawn from the market for commercial reasons, not for issues of safety or efficacy [8, 9]. This reminds us that hurdles like high upfront costs, long-term data collection, and navigating healthcare system reimbursement are very real challenges that we, as a community, must face together.
The Path Forward is a Shared Journey
The story of Beqvez is not an ending, but a vital chapter in an ongoing narrative of progress. Other gene therapies, like Hemgenix, are available, and the landscape is continuing to evolve with new non-factor therapies that work in entirely different ways to help the blood clot [10, 11].
As this new frontier of medicine unfolds, we are committed to providing you with clear, trustworthy information. We will celebrate the breakthroughs and be honest about the challenges. Your insights, your experiences, and your hope are what fuel this forward march. On this journey of discovery, you are not alone.
References:
- Srivastava, A., Khetan, V., & Das, S. K. (2019). Worry, Burden of Treatment, and Clinical Manifestations in Patients with Hemophilia A and B. Journal of Hemophilia Practice, 6(1), 32–40.
- Hoots, W. K., Pipe, S. W., & Lusher, J. M. (2020). Treatment of Hemophilia B: Practical Considerations from Infusions to Gene Therapy. European Journal of Haematology, 104(5), 439–449.
- Ljung, R. (2007). Haemophilia B. Orphanet Journal of Rare Diseases, 2(1), 50.
- Deng, F., Wang, Y., & Liu, P. (2023). Current advances in gene therapy for hemophilia B. Translational Pediatrics, 12(2), 325–334.
- Srivastava, A., Santagostino, E., Ducore, L. M., et al. (2020). Guidelines for the management of hemophilia. Haemophilia, 26(S6), 1–158.
- Perrin, G. Q., & George, L. A. (2022). Current and Emerging Gene Therapy Clinical Trials for Hemophilia. Hemophilia, 28(S1), 11–19.
- Pipe, S. W., Kim, J., Margaritis, H., et al. (2023). Fidanacogene elaparvovec gene therapy for hemophilia B. New England Journal of Medicine, 388(10), 936–948.
- Pfizer. (2023, December 14). Pfizer Statement on Hemophilia B Gene Therapy Program.
- Miller, P. (2023, December 14). Pfizer walks away from hemophilia B gene therapy Beqvez, citing competitive landscape, market access hurdles. Fierce Pharma.
- CSL Behring. (2023, February 17). CSL Behring Announces European Commission Approval of HEMGENIX® (etranacogene dezaparvovec) for Adults with Severe and Moderately Severe Hemophilia B.
- Pipe, S. W., & Angchaisuksiri, P. (2023). Emerging Non-Factor Therapies in Hemophilia: An Update. Journal of Clinical Medicine, 12(7), 2617.