For so many families in our community, the journey with an ultra-rare disease can feel isolating. It often begins with a long, frustrating search for answers, a “diagnostic odyssey,” and leads to managing complex symptoms without a clear path forward. We know this journey can be challenging, and we want you to know that you are not alone. Today, we want to walk with you through a story of incredible scientific progress that brings a new chapter of hope for one such condition: WHIM syndrome.[1]
In this discussion, we’re going to take a deeper look at this rare disorder and the development of the first-ever therapy designed specifically to address its root cause. Now, we know that the science can sometimes feel complex, but our goal is to break it down together.
What is WHIM Syndrome? A Story in an Acronym
WHIM syndrome is an extremely rare genetic disorder, and its name is an acronym that tells the story of its main features:[1]
- Warts (severe and persistent)
- Hypogammaglobulinemia (low levels of antibodies in the blood)
- Infections (recurrent, especially bacterial ones)
- Myelokathexis (a condition where key immune cells get trapped in the bone marrow)[2]
That last part, Myelokathexis, is at the very heart of the problem.[2] Imagine your body’s best infection-fighters—white blood cells called neutrophils and lymphocytes—are all trained and ready for a fight. But, instead of being out in your bloodstream protecting you, they’re stuck behind a locked door in the bone marrow, unable to get to where they’re needed most.[3] This leaves the body vulnerable to the infections and other issues that define WHIM syndrome.
The Root of the Challenge: An Overactive “Doorbell”
So, what’s keeping that door locked? In 2003, researchers made a breakthrough discovery; WHIM syndrome is caused by a mutation in a gene called CXCR4.[4] This gene provides the instructions for a receptor that acts like a cellular “doorbell.” Its job is to receive a signal (from a molecule called CXCL12) that tells white blood cells to stay within the safe confines of the bone marrow.[5]
In a healthy person, this doorbell rings, and then it quickly turns off, allowing the cells to leave when they’re needed. But in WHIM syndrome, the mutation causes a “gain-of-function,” meaning the doorbell is overactive—it’s stuck in the “on” position.[6] It constantly signals for the cells to stay put, which is why they become trapped. This single genetic typo is the direct cause of the low white blood cell counts that lead to the infections and other symptoms of WHIM.
A New Chapter of Hope: A Targeted Therapy
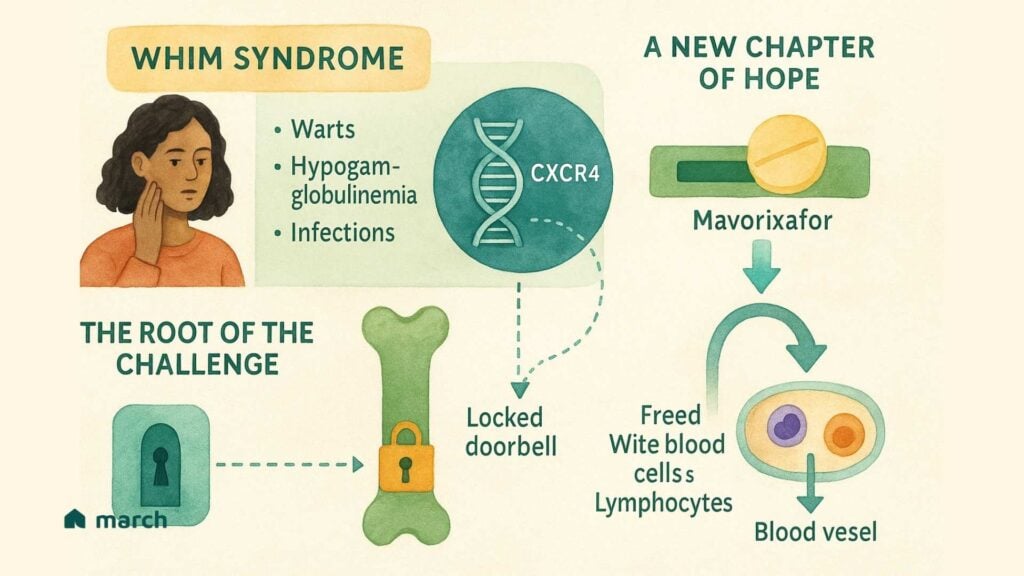
For decades, managing WHIM syndrome meant treating the symptoms, using antibiotics for infections or immunoglobulin infusions to supply missing antibodies.[1] These are incredibly important tools, however, they don’t fix the underlying problem of the locked door.
This is where a new therapy called mavorixafor (XOLREMDI®) represents such a significant step forward. Approved by the FDA in April 2024 for patients 12 and older, mavorixafor is the first treatment designed to directly target the overactive CXCR4 doorbell.[7]
Here’s how we can think about it working:
Mavorixafor acts like a temporary cover for the doorbell. It’s an oral medication that blocks the receptor, preventing it from receiving the constant “stay put” signal.[8] By interrupting this signal, it essentially “unlocks the door,” allowing the trapped neutrophils and lymphocytes to be released from the bone marrow into the bloodstream.[7]
Consequently, in the pivotal clinical trial, this led to a significant increase in the number of these crucial immune cells circulating in the blood. Most importantly, a 60% reduction in the rate of severe infections for those taking the therapy compared to a placebo. [9]
Hope, Realism, and the Road Ahead
The approval of a targeted therapy for an ultra-rare disease is a monumental achievement, and it offers real, tangible hope to our community. It’s a testament to the patients, families, researchers, and advocates who have worked for years to make this progress possible.
This is a huge step, but it’s not the end of the journey. In its main trial, the therapy didn’t show a significant improvement in warts, and its approval is currently for patients 12 years and older, highlighting a need for continued research for younger children.[9][10]
Marching Forward
This is what forward movement looks like, one dedicated, meaningful step at a time. It’s a step that opens the door for more research and inspires the development of future therapies, including potential cures inspired by the incredible story of a patient who was spontaneously cured of WHIM.[11]
As we watch these new treatments become a reality, we are committed to being here to support you with clear, trustworthy information. We will continue to celebrate the breakthroughs and be open about the challenges that remain. Your journey matters, and you are not alone.
To learn more about the science behind new treatments, we encourage you to listen to our podcast episode that delves into the history and science of gene editing.
Sources
[1] National Organization for Rare Disorders (NORD). (n.d.). WHIM Syndrome.
[2] Zuelzer, W. W. (1964). “Myelokathexis”–a new form of chronic granulocytopenia. New England Journal of Medicine.
[3] Dale, D. C., et al. (2020). The CXCR4 antagonist mavorixafor in patients with WHIM syndrome. Blood.
[4] Hernandez, P. A., et al. (2003). Mutations in the chemokine receptor gene CXCR4 are associated with WHIM syndrome, a combined immunodeficiency disease. Nature Genetics.
[5] X4 Pharmaceuticals. (2024). XOLREMDI (mavorixafor) Prescribing Information.
[6] Balabanian, K., et al. (2005). WHIM syndromes with different genetic anomalies are accounted for by impaired CXCR4 desensitization and internalization. Blood.
[7] Badalato, R., et al. (2024). Mavorixafor for treatment of WHIM syndrome: a phase 3, randomised, placebo-controlled trial. The Lancet.
[8] McDermott, D. H., et al. (2019). The CXCR4 antagonist plerixafor corrects panleukopenia and prevents bacterial infections in WHIM syndrome. Blood.
[9] X4 Pharmaceuticals. (2024, April 29). X4 Pharmaceuticals Announces U.S. FDA Approval of XOLREMDI™ (mavorixafor). [Press Release].
[10] U.S. Food & Drug Administration (FDA). (2024). Novel Drug Approvals for 2024.
[11] McDermott, D. H., et al. (2015). Chromothriptic cure of WHIM syndrome. Cell.
